Classes of atoms
The following pictures show
how the density deformations support the chemical notion of
classes of atoms.
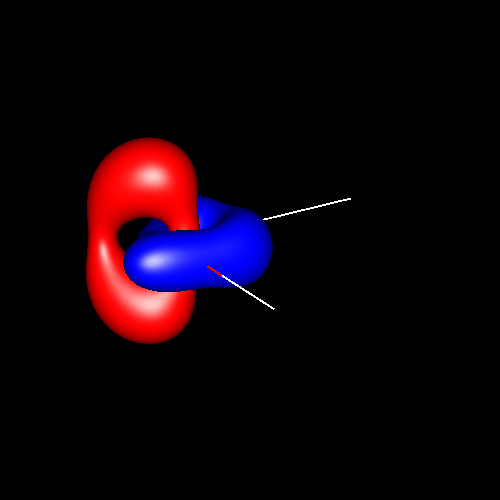
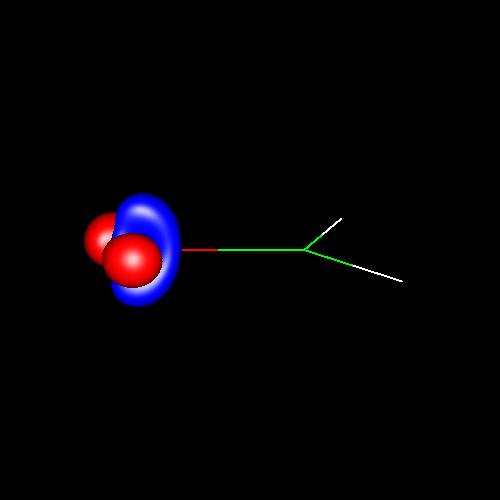
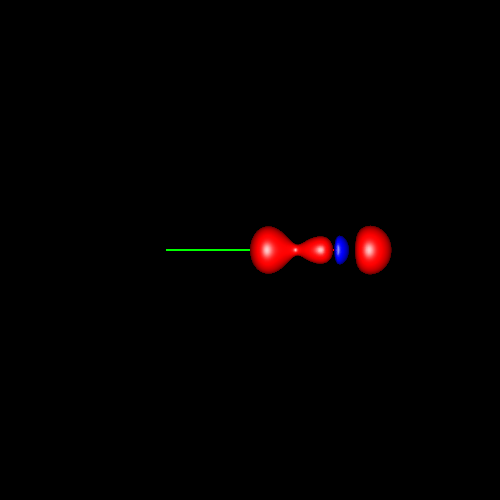
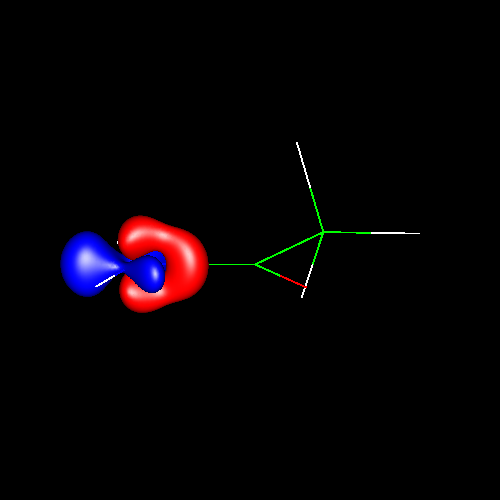
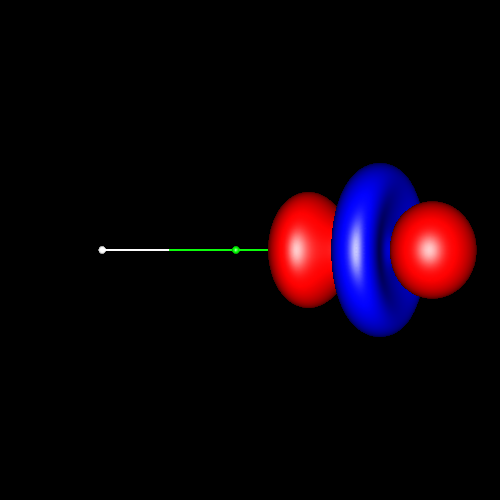
In the first
three pictures, contour surfaces of values ±0.075 au
corresponding to oxygen
deformations in water, formaldehyde and carbon
monoxide have been depicted. In water, the deformations can be
associated to its two lone pairs, which extend above and below the
molecular plane and are connected to each other. In formaldehyde, the
charge accumulations associated to the two lone pairs are not connected
and are placed in the molecular plane. Finally, in carbon monoxide the
shape of the charge concentrations are completely different from the
previous ones, and those lying outside of the bond region and close to
oxygen suggest the existence of a single lone pair there.
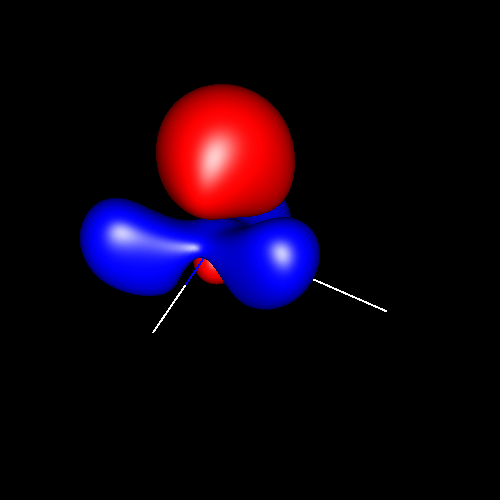
The next pictures show the contour surfaces of values ±0.025 au corresponding to the deformations of nitrogen in ammonia, acetamide and hydrogen cyanide:
The next pictures show the contour surfaces of values ±0.025 au corresponding to the deformations of nitrogen in ammonia, acetamide and hydrogen cyanide:
Click on the figures to enlarge, click here for further pictures.