Density deformations: definitions and conventions
In this analysis, we use the atomic deformations defined as the (pseudo-) atomic densities minus their spherical terms:
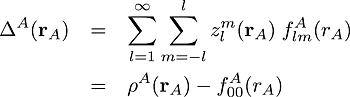
(1)
and the molecular deformation which is the sum of all the atomic deformations: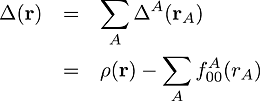
(2)
This latter quantity has some similarity with the so-called charge density difference functions, which are the differences between the molecular density and the densities
of the isolated atoms, placed in the corresponding sites of the molecule and taken in suitable valence states.Note, however, that the spherical terms subtracted in eq(2) are obtained from the very molecular density, without any intervention of reference atoms. In practice, these two quantities exhibit some resemblance, but this is because, as it will be shown in the following section, the spherical terms of the atoms in our representation of the molecular density are similar to those of the isolated atoms.
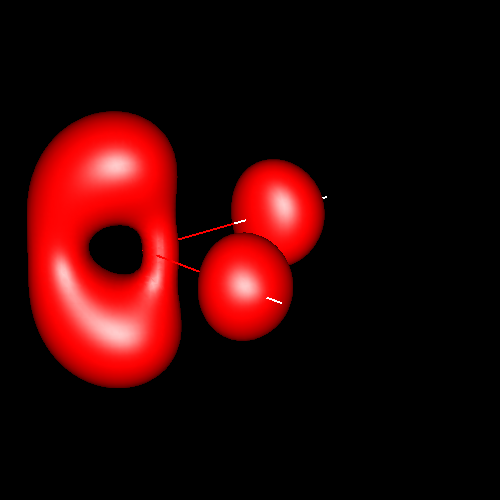
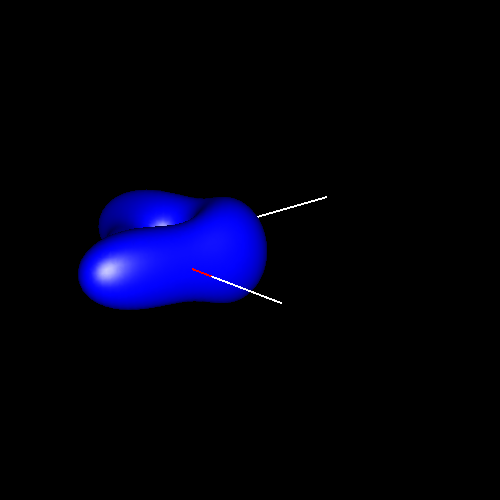
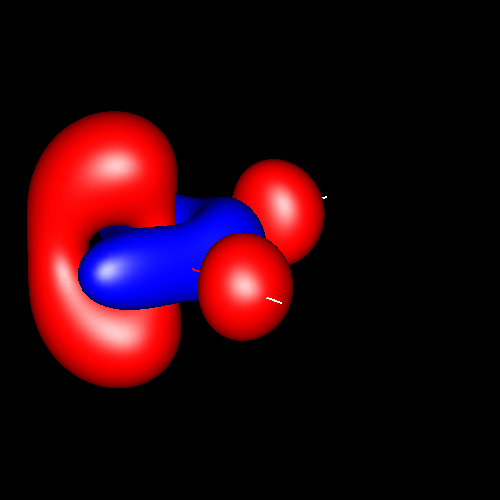
For convention, in pictures positive density deformations (charge accumulations) will be drawn in red, and negative deformations (charge depletion), in blue. Both types of deformations for a given absolute contour value will be drawn together in a single picture, except in those cases in which a separation of positive and negative deformations in diferent figures is necessary for clarity. Different absolute contour values will be drawn in different pictures. The next pictures illustrate these conventions in case of water for contour values of ±0.05 au (electron / bohr3).