Single, double and triple bonds
Single bonds are characterized by charge accumulations of nearly cylindrical symmetry between bonding atoms. As representative of these kind of bond, the following pictures show the contour surface of the density deformations of methane and ethane for contour values of ±0.025 au. (charge accumulation, in red; charge depletion, in blue). Analogous deformations can be seen in almost all the molecules of this study.
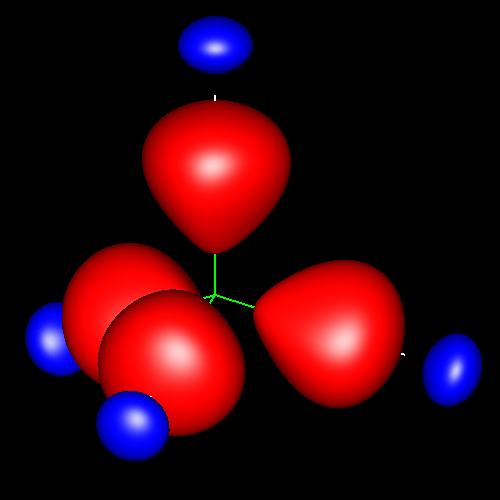
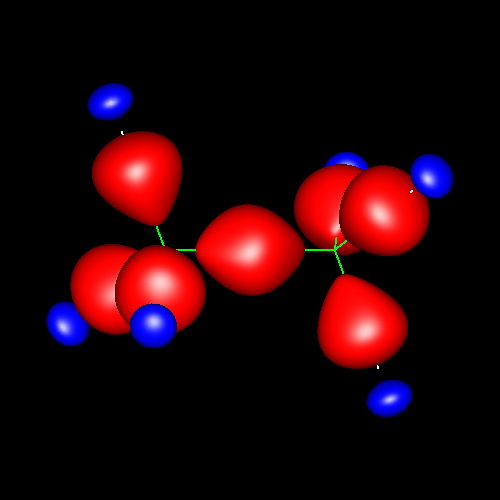
Click on the figures to enlarge, click here for further pictures.
Double bonds are characterized by strong charge accumulations with upward and downward distortions which destroy the cylindrical symmetry. This asymmetry can be identified with the pi character (ellipticity). Two simple examples can be found in ethylene and allene, whose deformations corresponding to ±0.025 au can be appreciated in the following pictures. Notice the charge depletion above and below the molecular plane in the vicinity of carbons (in blue).



Click on the figures to enlarge, click here for further pictures.
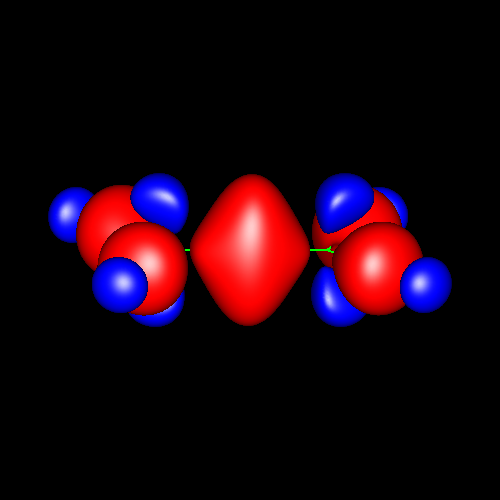
Triple bonds are characterized by a strong charge accumulation of cylindrical symmetry accompanied by two perpendicular rings of charge depletion centered in the bonding atoms.
Acetylene and hydrogen cyanide are representative cases whose deformations corresponding to ±0.025 au are shown in the following pictures.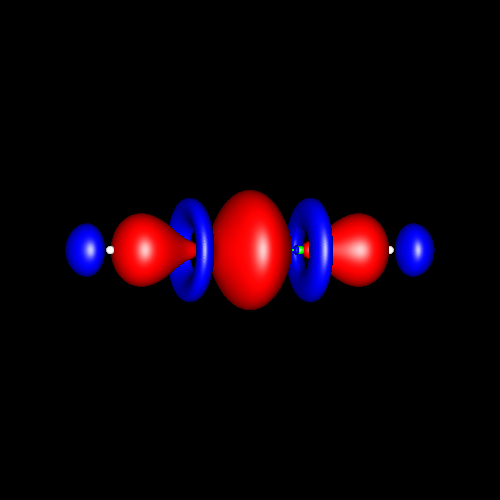
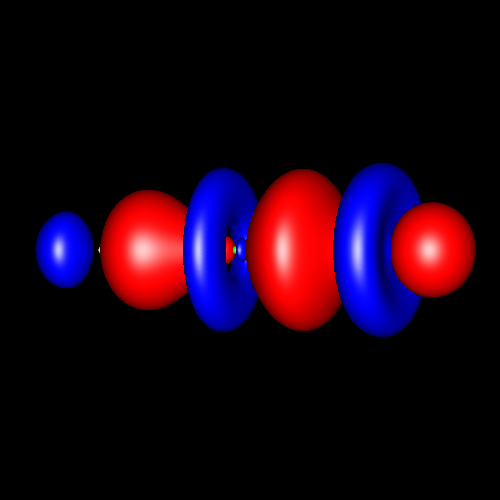
Click on the figures to enlarge, click here for further pictures.
Cuerpo de la página.