Single, double and triple bonds
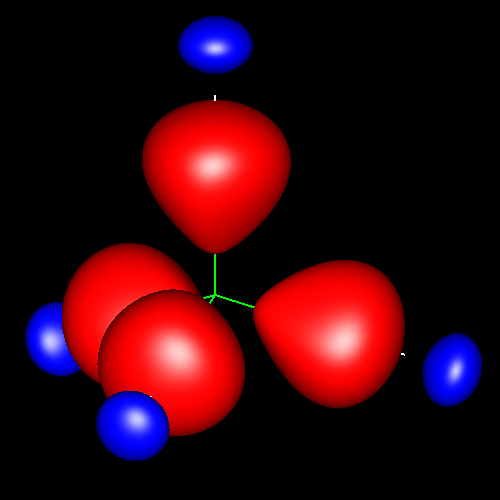
Single bonds are characterized by charge accumulations of nearly cylindrical symmetry between bonding atoms. As representative of these kind of bond, the following pictures show the contour surface of the density deformations of methane and ethane for contour values of ±0.025 au. (charge accumulation, in red; charge depletion, in blue). Analogous deformations can be seen in almost all the molecules of this study.
 |
 |
 |
Click on the figures to enlarge, click here for further pictures.
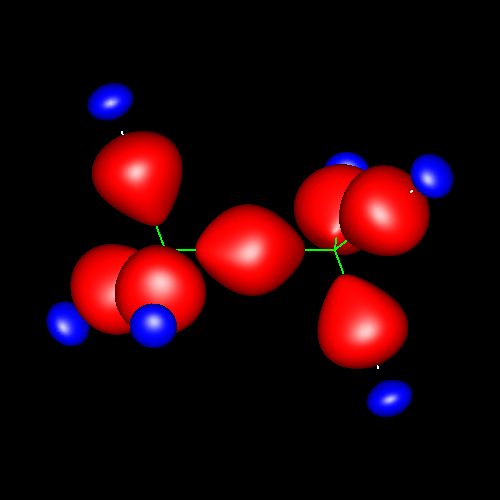
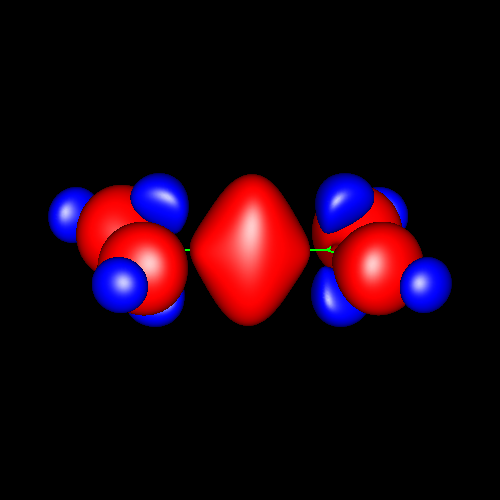
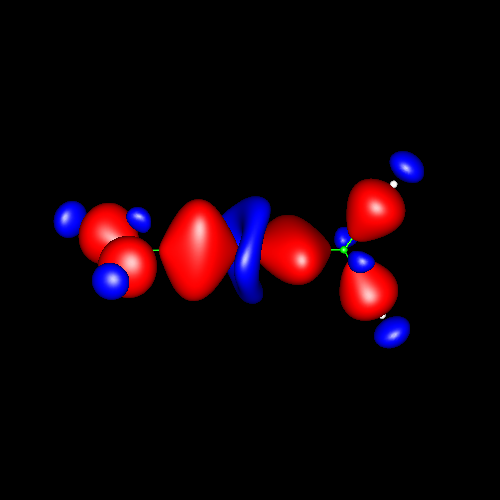
Double
bonds are characterized by strong
charge accumulations with upward and downward distortions which
destroy the cylindrical symmetry. This asymmetry can be identified with
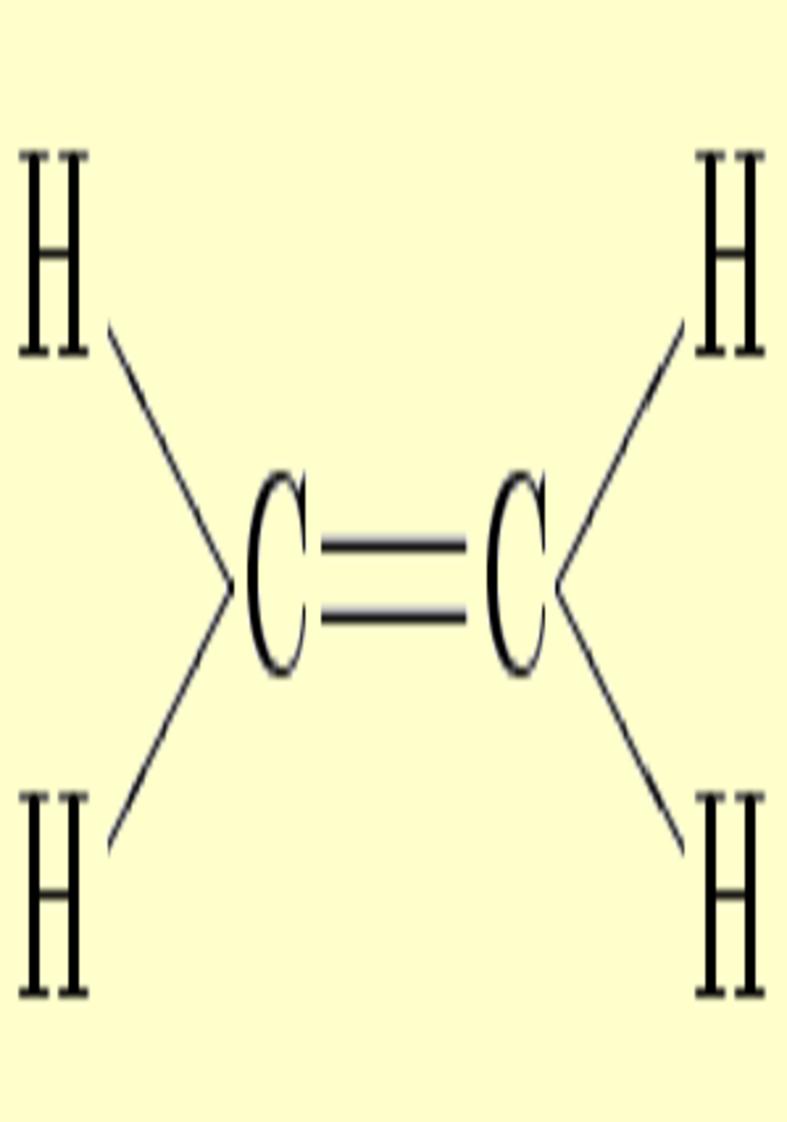
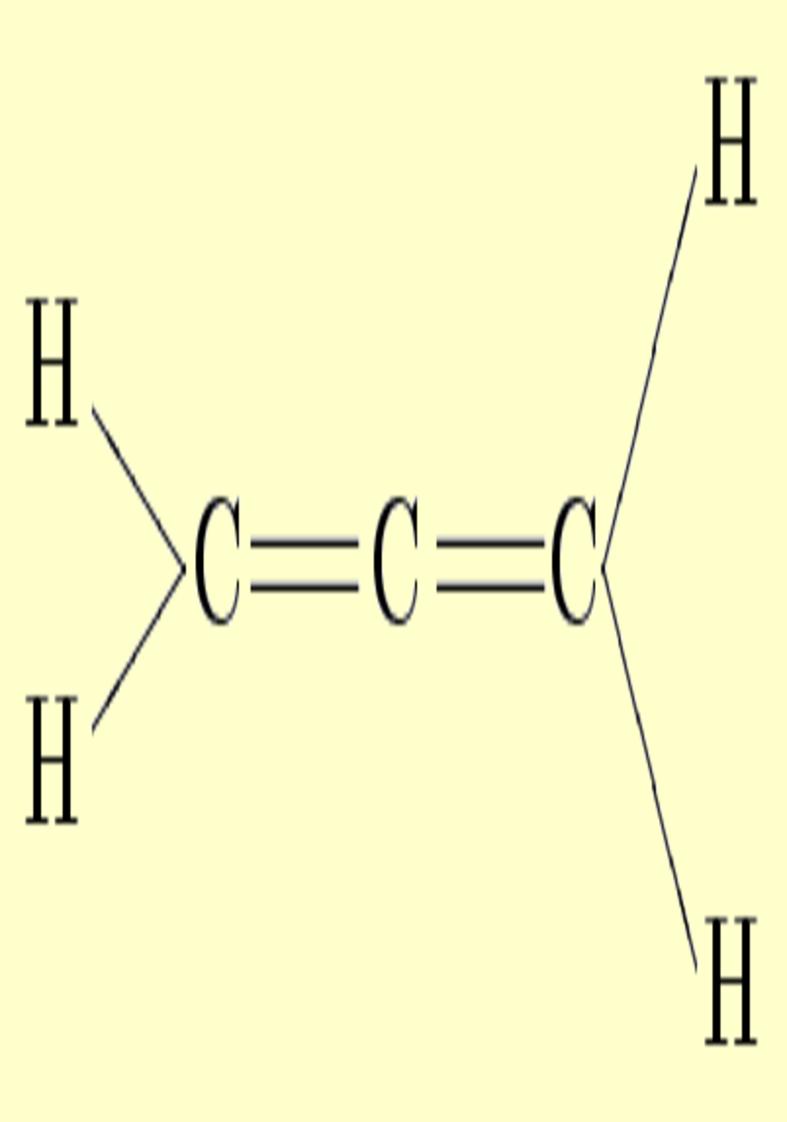
the pi character (ellipticity). Two simple examples can be found in ethylene and allene,
whose deformations corresponding to ±0.025 au can be appreciated
in the following pictures. Notice the charge depletion above and below
the molecular plane in the vicinity of carbons (in blue).
 |
 |
 |
 |
Click
on the figures to enlarge, click here for
further pictures.
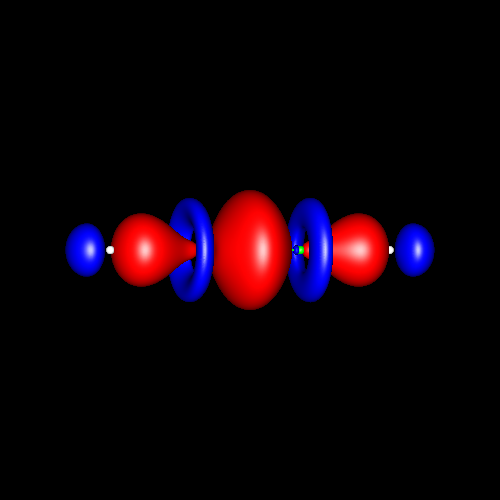
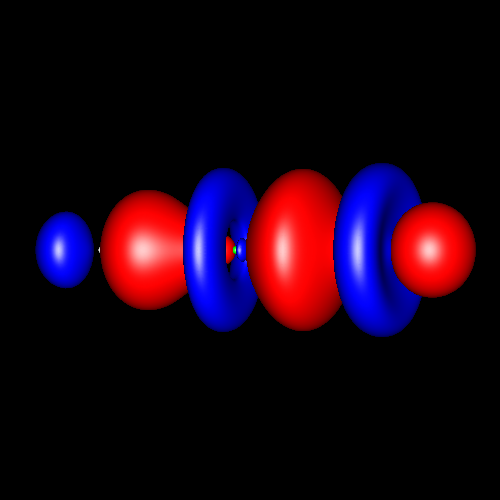
Triple
bonds are characterized by a strong
charge accumulation of cylindrical symmetry accompanied by two
perpendicular rings of charge depletion centered in the bonding atoms. Acetylene and hydrogen
cyanide are representative cases whose deformations corresponding
to
±0.025 au are shown in the following pictures.