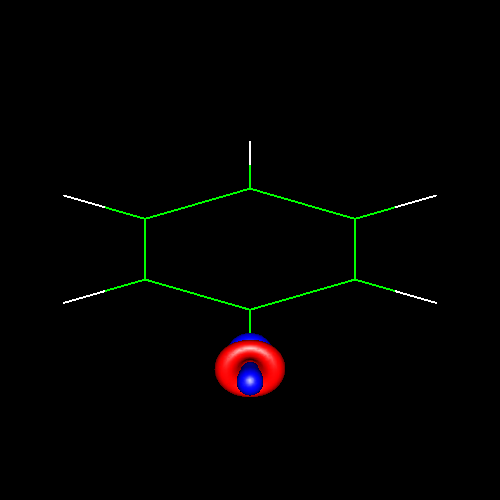
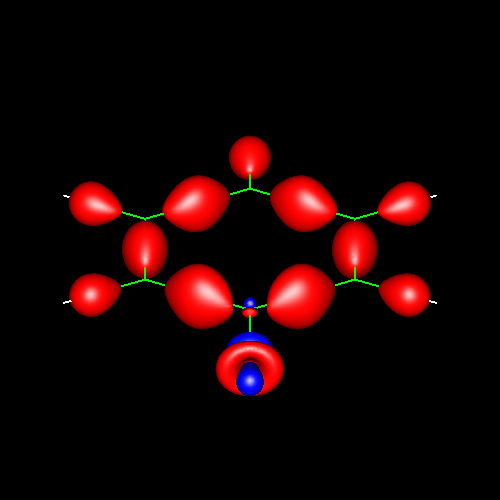
Lone pairs
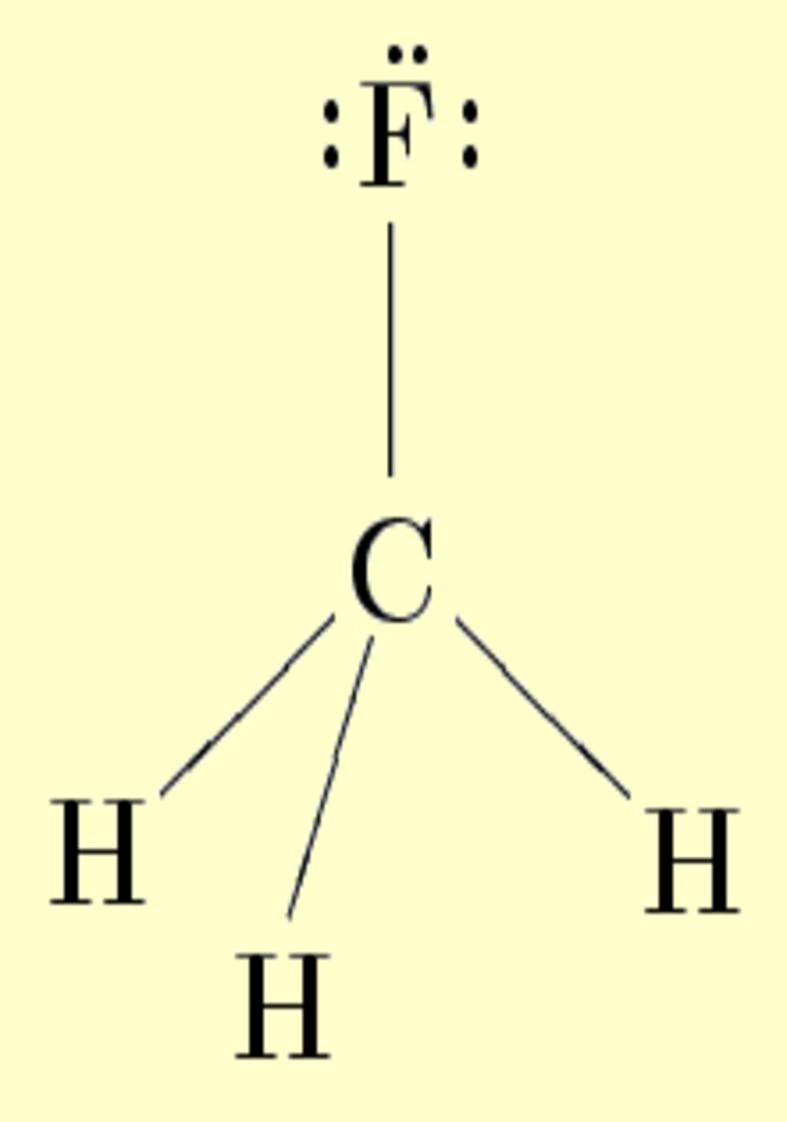
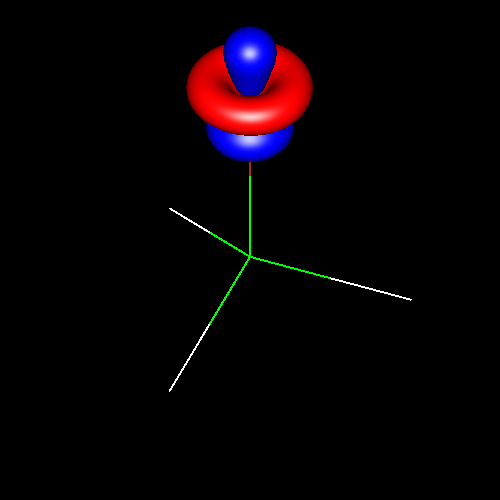
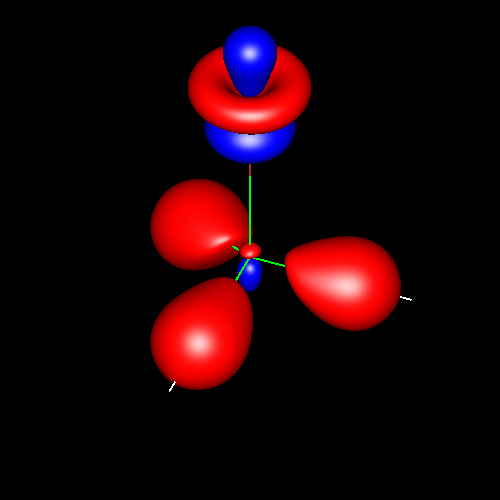
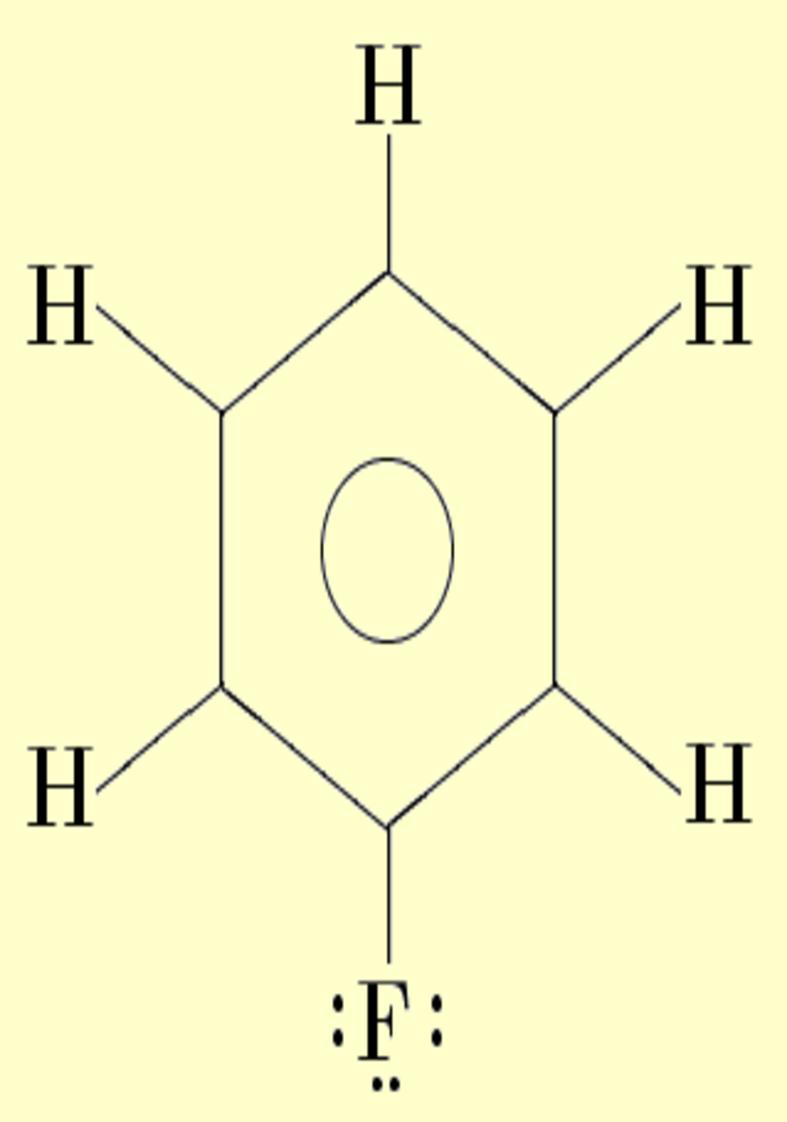
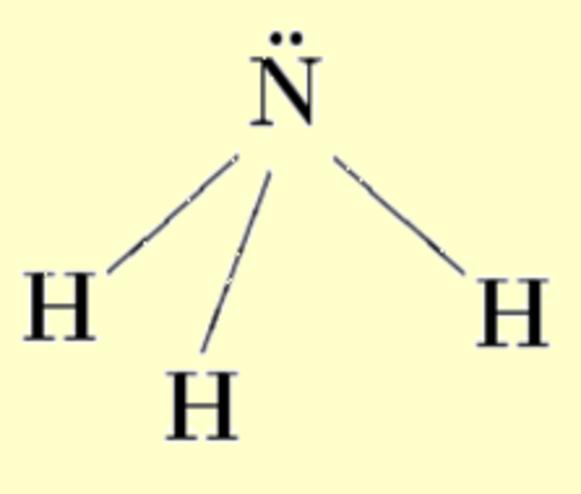
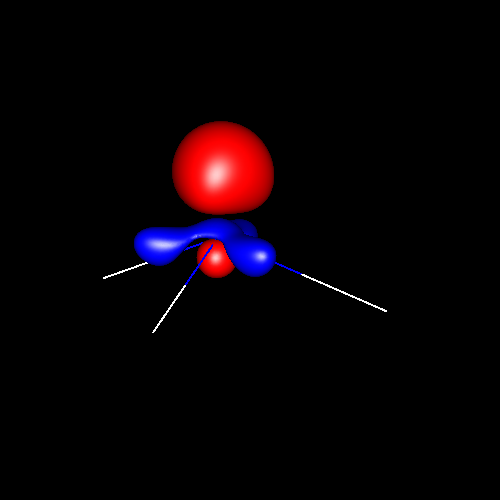
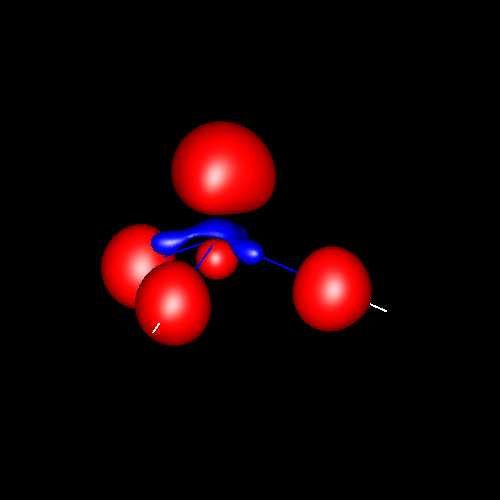
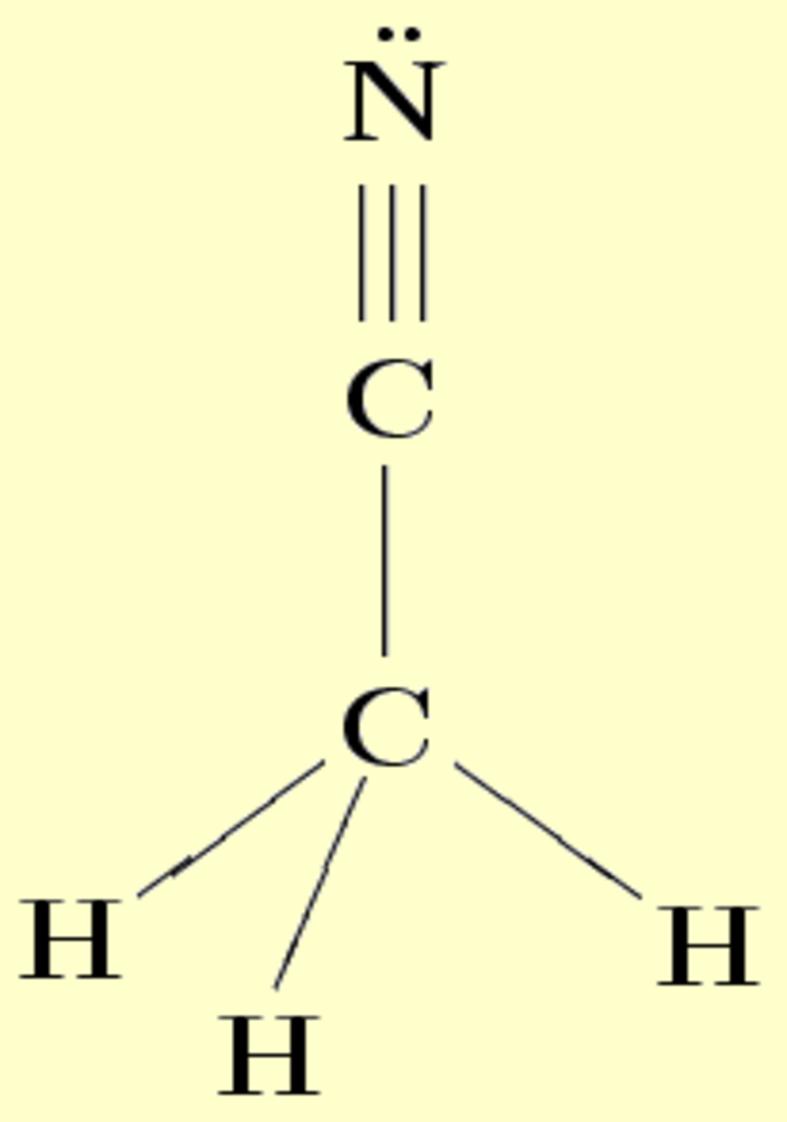
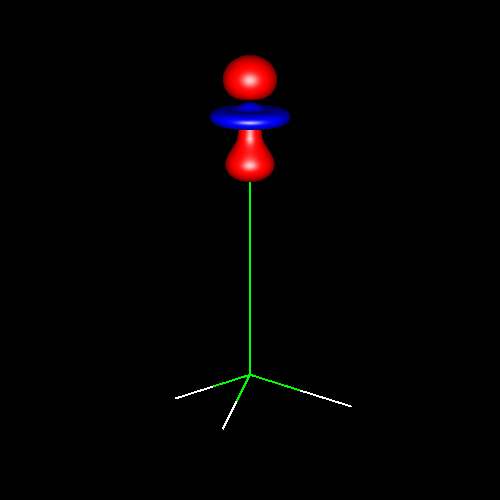
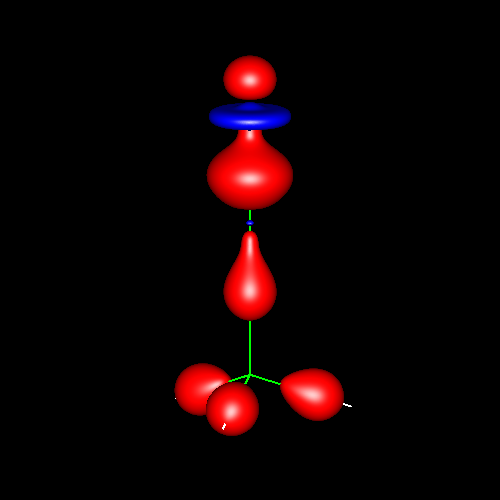
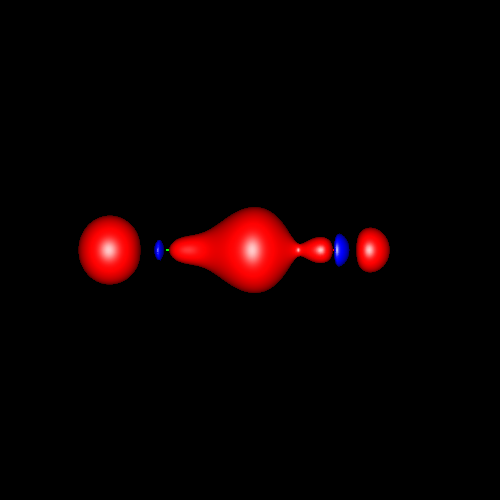
Lone pairs compose a beautiful example of the close relationship between the chemical notions and the density deformations. In the upper figures of the following table, the Lewis structure ofammonia (left plate) is compared with the deformations of nitrogen (middle plate) and the total molecular deformations (right plate) for contour values of ±0.05au. As it can be seen, beside the three charge accumulations corresponding to the sigma N--H bonds, there is a large, and nearly spherical, charge accumulation in the opposite part of the N atom. This structure is reproduced practically unchanged in amines. A diferent type of charge accumulation appears in nitriles, as it can be observed in case of ethanonitrile (lower figures).
| Lewis
structure |
Nitrogen deformation (±0.05au) | Molecular deformation (±0.05au) |
 |
 |
 |
 |
 |
 |
Click on the figures to enlarge, click here for further pictures.
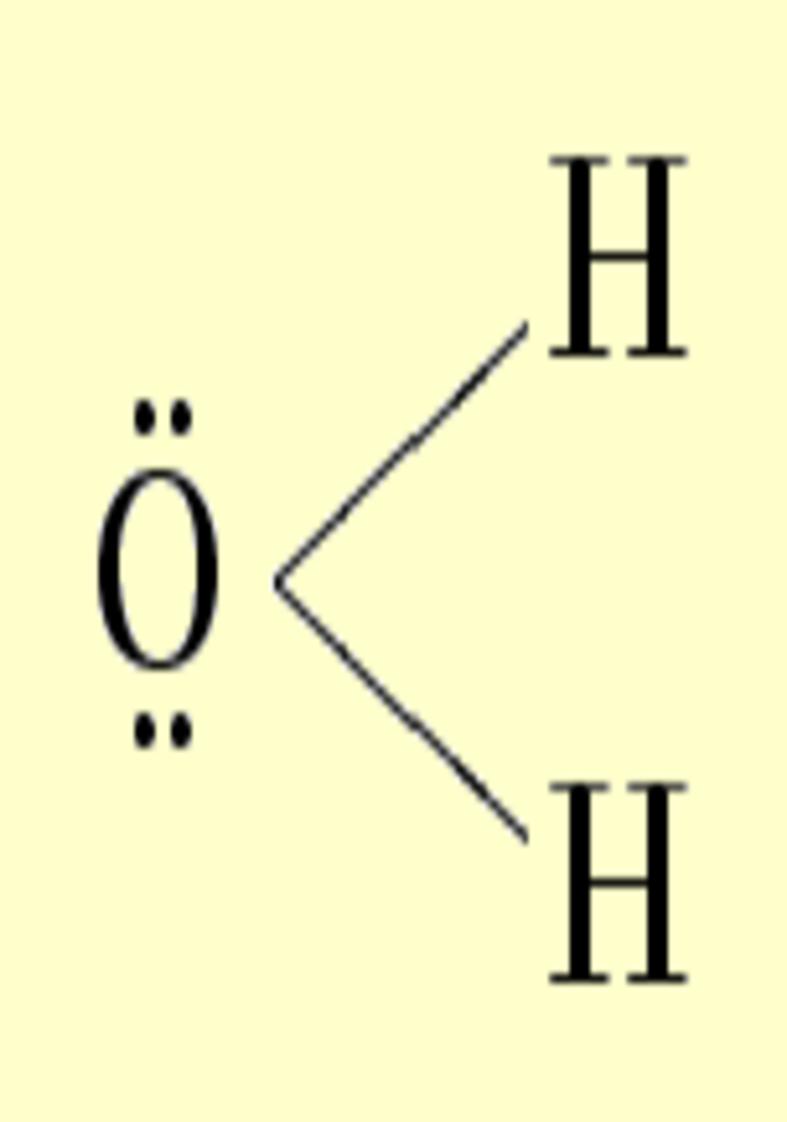
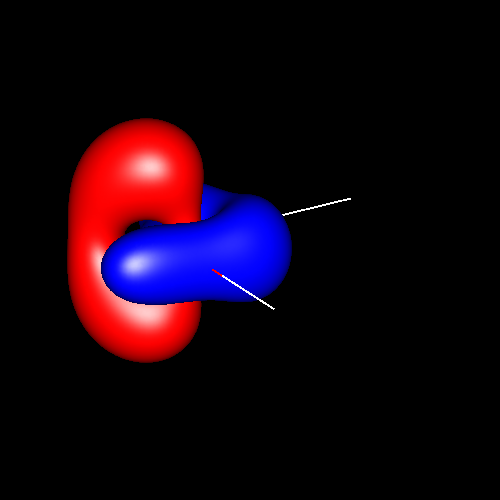
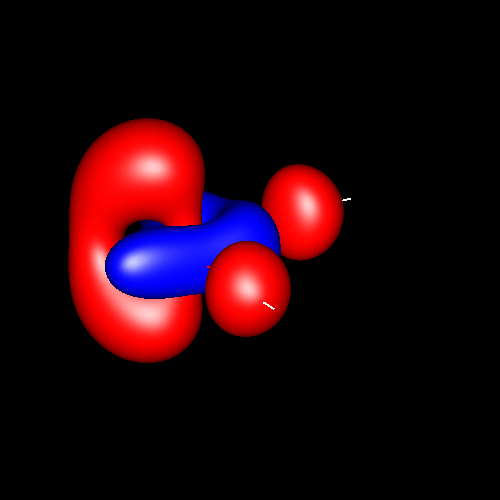
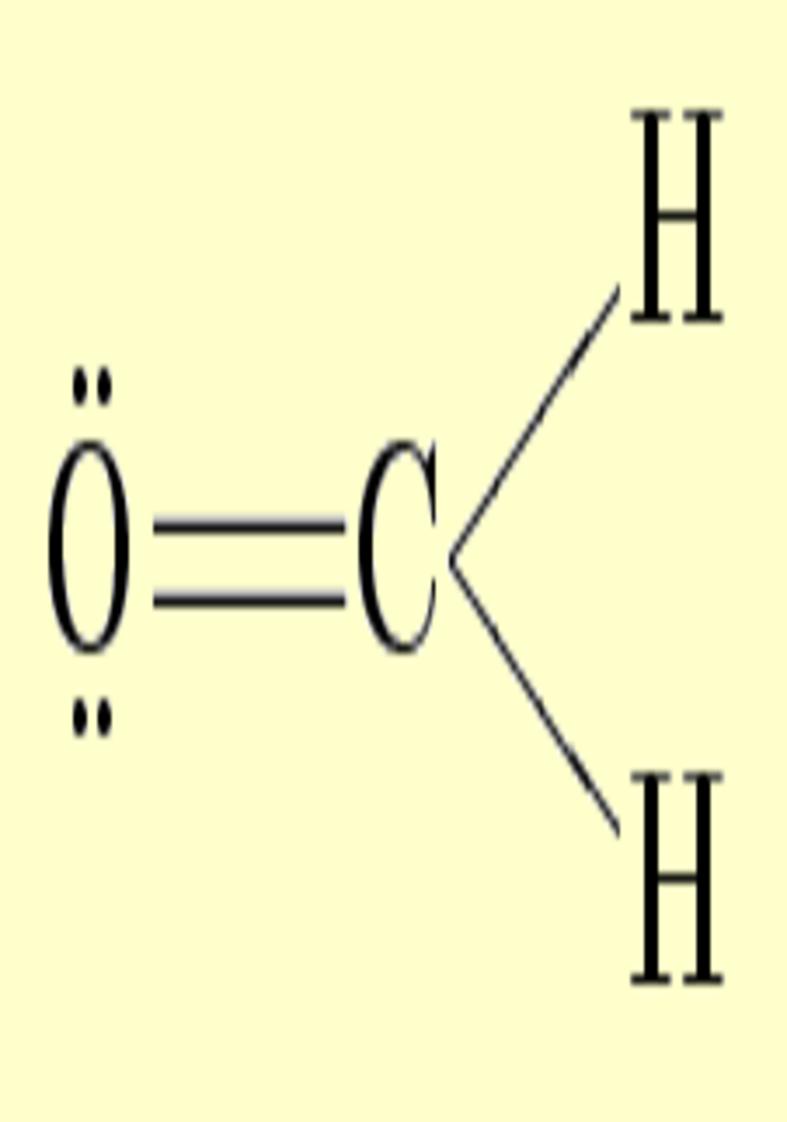
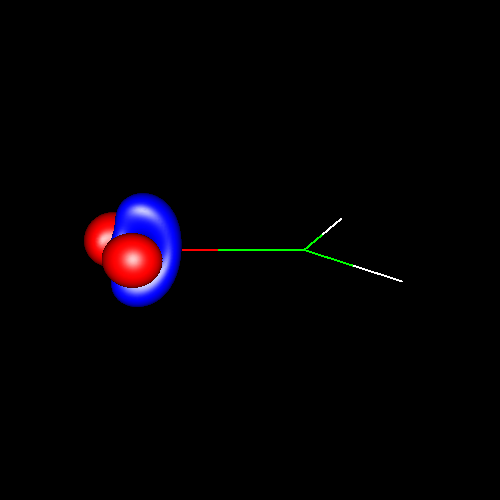
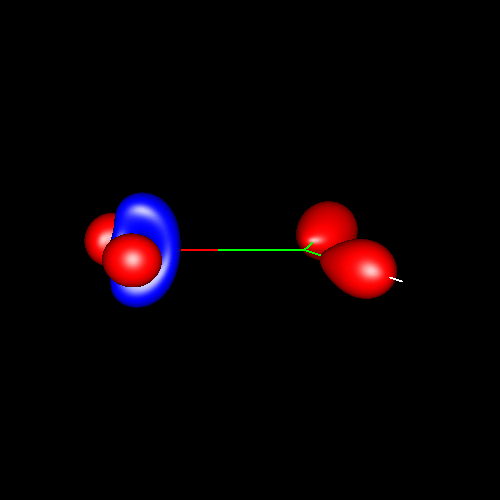
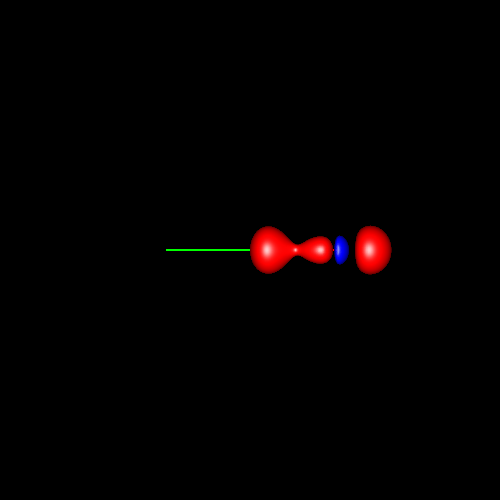
Another interesting case is
the atom of oxygen. Depending on the molecular
environment, different types of deformations associated to lone pairs
can be observed. The following figures show the Lewis
structures and the deformations in water,formaldehyde
and cabon monoxide for a contour value of
±0.05.
| Lewis structure | Oxygen deformation (±0.05au) | Molecular deformation (±0.05au) |
 |
 |
 |
 |
 |
 |
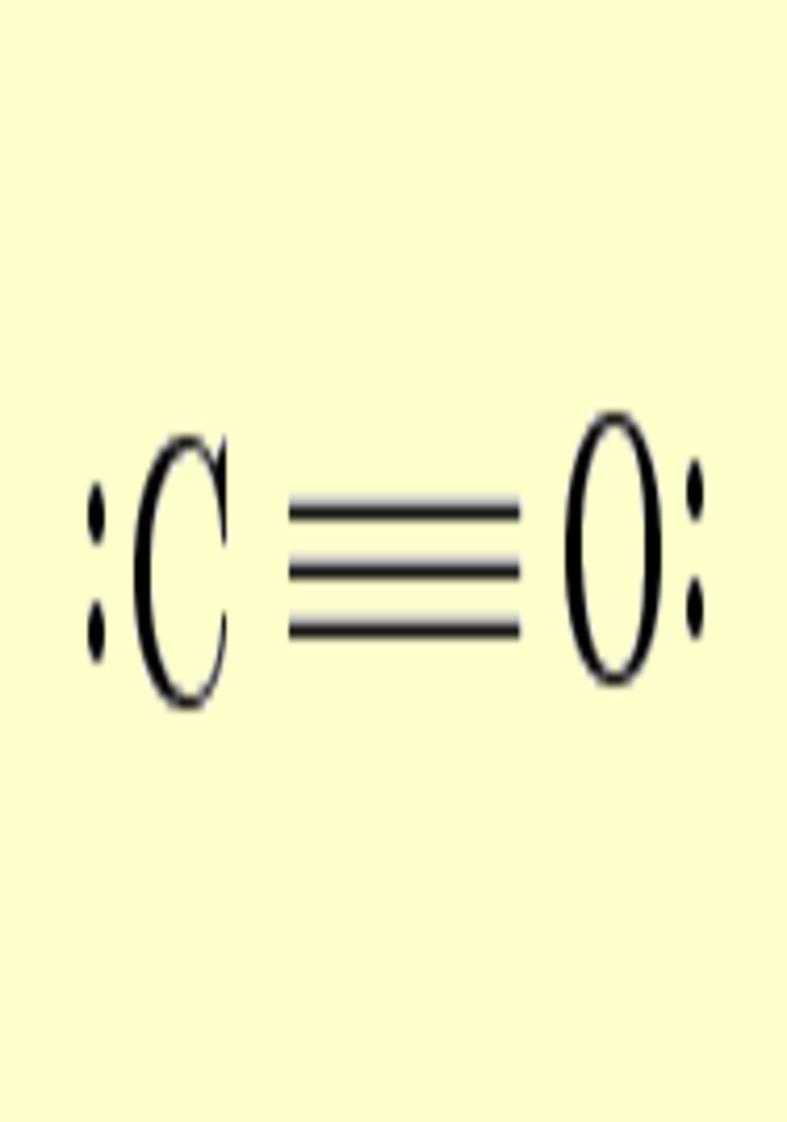 |
 |
 |