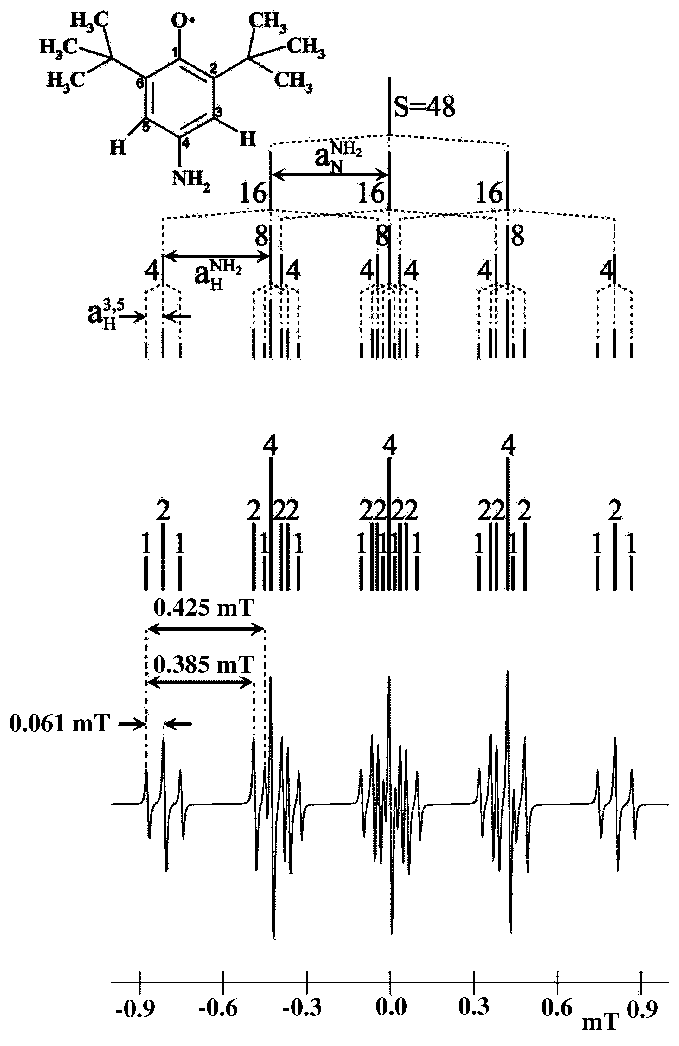
 |
 |
There are three hyperfine splittings. The two largest are very close and, therefore, there is an overlap of the central lines of its triplets. The intensities suggest that the triplet corresponds to the coupling between the unpaired electron with the nitrogen atom, lines 6, 14 and 22.
Hyperfine splittings:
![]() = 0.061 mT (triplet, 1:2:1). Between 1
= 0.061 mT (triplet, 1:2:1). Between 1![]() and 2
and 2![]() line.
line.
![]() = 0.385 mT (triplet, 1:2:1). Between 1
= 0.385 mT (triplet, 1:2:1). Between 1![]() and 4
and 4![]() line.
line.
![]() = 0.425 mT (triplet, 1:1:1). Between 1
= 0.425 mT (triplet, 1:1:1). Between 1![]() and 5
and 5![]() line.
line.
Maximum number of lines (Eq. (9)):
N = 3 ![]() 3
3 ![]() 3 = 27 lines.
3 = 27 lines.
Length of the spectrum (Eq. (8)):
L = 0.061 ![]() 2 + 0.385
2 + 0.385 ![]() 2 + 0.425
2 + 0.425 ![]() 2 = 1.742 mT.
2 = 1.742 mT.
Reconstruction: It is presented in the upper part of the Fig. 27. The numbers on the lines show their relative intensities.
This radical is a resolved example, try to understand it. Load the simulator and introduce the data corresponding to this spectrum to obtain a correct simulation.
NOTES: Here it is not possible to assign the hyperfine splitting of the protons from the spectrum. However, there are different procedures to do that assignation:
The nine spectra presented below (subsections 8.2.2 a 8.2.11) are unresolved. They have 2, 3 or 4 groups of equivalent nuclei with different value of I. Look carefully at the molecule structure to find out the number of hyperfine splittings and the nuclei that originates those splittings with the unpaired electron. Assign, if possible, the order (multiplicity) of the hyperfine splittings. For the interpretation, proceed like in section 8.1.2. Use the length of the experimental spectrum as an additional information to get the last hyperfine splitting using the Eq. (8). Print out the simulated and experimental spectra and the data used in the simulation.