


Next: 8 Spectra of Organic
Up: EPR Tutorial
Previous: 6 Radical spectra with
Contents
7 Basic Rules for the interpretation of EPR spectra
The following rules are applicable to spectra that contain only one radical,
they are not validate for mixtures of two or more radicals.
- The position of the lines of a spectrum is symmetric with respect to the central point of the spectrum.
- When a spectrum does not present an intense line in its centre
it suggests that there exist an odd number of equivalent nuclei
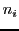 with half integer spin
(
with half integer spin
(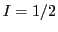 ,
, 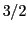 ,
, 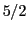 ,
,  ).
Nevertheless, the presence of an intense central line does not exclude the existence of
an odd number of nuclei,
because that line can be make by an accidental relation between the hyperfine splittings
).
Nevertheless, the presence of an intense central line does not exclude the existence of
an odd number of nuclei,
because that line can be make by an accidental relation between the hyperfine splittings 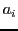 .
.
- The total length of a spectrum (separation in mT between the two most external lines) is given by:
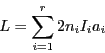 |
(8) |
being 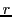 the number of groups of equivalent nuclei and
the number of groups of equivalent nuclei and 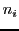 the number of equivalent nuclei with
hyperfine splitting
the number of equivalent nuclei with
hyperfine splitting 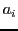 and quantum number of spin
and quantum number of spin 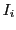 .
.
- The maximum number of lines is given by:
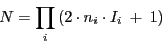 |
(9) |
where 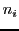 represents the number of nuclei with spin
represents the number of nuclei with spin 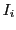 .
.
- The sum of the relative intensities of all lines (total number of transition) is:
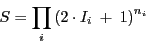 |
(10) |
- The distance between the two first lines of the spectrum correspond to the smaller hyperfine splitting.
The second hyperfine splitting is the distance between the first line and the following not identified one.



Next: 8 Spectra of Organic
Up: EPR Tutorial
Previous: 6 Radical spectra with
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada