


Next: 8.2.7 1,4-dihydro pyrazine cation
Up: 8.2 Radicals containing r
Previous: 8.2.5 Diethyl amine cation
Contents
The substitution of hydrogen by deuterium in the amine group (NH) of the cation radical [a026]
will produce the following effects:
a) an increase in the number of theoretical lines from
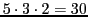 to
to
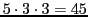 ;
b) a decrease in the hyperfine splitting
;
b) a decrease in the hyperfine splitting
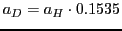 mT and
c) a reduction in the length of the spectrum.
mT and
c) a reduction in the length of the spectrum.
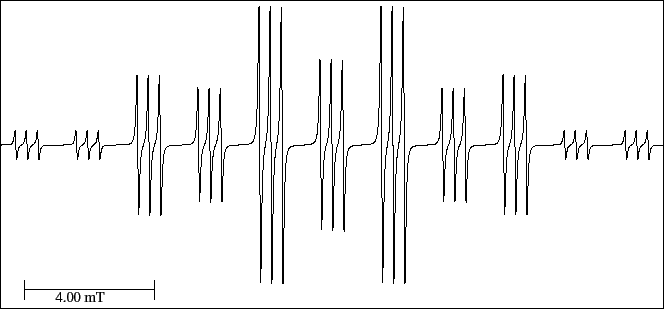
With these considerations, interpret the spectrum (Fig. 33);
load the EPR simulator and measure the hyperfine splittings of each nucleus.
Compare its length with that of the undeuterated radical.
Figure 33:
EPR spectrum of the mono-deuterium diethyl amine cation radical.
 |
[Exercise]
- Print the spectrum of Fig. 33
by pressing the following link
- Reconstruct the spectrum in the graph paper of the form-a027 by successive splittings.
- Indicate the relative theoretical intensities of each multiplet.
- Number the lines of the spectrum; mark which ones add intensities; and
indicate the relative theoretical intensity.
- Pay attention to the scale and do the transformation from cm to mT.
- Measure and assign the three hyperfine splittings; measure also the length of the spectrum.
- Complete the Table of hyperfine splittings (tab-a026) of the previous section 8.2.5.



Next: 8.2.7 1,4-dihydro pyrazine cation
Up: 8.2 Radicals containing r
Previous: 8.2.5 Diethyl amine cation
Contents
Universidad Autónoma de Madrid, Departamento de Química Física Aplicada